Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Analytical Sciences, 39(9), p.1575 - 1583, 2023/09
Times Cited Count:0 Percentile:0(Chemistry, Analytical)Extraction of Rh from HCl can be performed by NTAamide(C6) (hexahexyl-nitrilotriacetamide) and other related compounds into n-dodecane. We use ion-pair extraction of anionic species of Rh-chloride and protonated extractant. Rh behave as anion in hydrochloric acid and the tertiary nitrogen atom in extractant may be protonated to produce the quaternary amine in acidic condition. From the present work, the maximum distribution ratio of Rh(III) is 16. The D(Rh) values are changeable during preparation of the aqueous solutions because different Rh-Cl-H O complexes are formed in HCl media and show the slow exchange rate between Cl and H
O complexes are formed in HCl media and show the slow exchange rate between Cl and H O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl
O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl (H
(H O)
O) and RhCl
and RhCl (H
(H O)
O)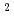
 exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
Sasaki, Yuji; Morita, Keisuke; Ito, Keisuke; Suzuki, Shinichi; Shiwaku, Hideaki; Takahashi, Yuya*; Kaneko, Masaaki*; Omori, Takashi*; Asano, Kazuhito*
Proceedings of International Nuclear Fuel Cycle Conference (GLOBAL 2017) (USB Flash Drive), 4 Pages, 2017/09
no abstracts in English
Kaneko, Masashi; Watanabe, Masayuki; Suzuki, Hideya; Matsumura, Tatsuro
no journal, ,
Nitrilotriacetamide (NTAamide) is considered to be a candidate for the separation reagent of minor-actinides (MA) from lanthanides (Ln) for an application to partitioning and transmutation. We have elucisdated the separation mechanism of Am from Eu with NTAamide by means of density functional theory (DFT). The present study applied the DFT method to Ln pattern of distribution ratio with NTAamide by solvent extraction. The extracted complex by NTAamide was modeled as [Ln(NTAamide) ]
] (Ln = La, Ce, Nd, Sm, Eu, Gd) by referring the analogous crystal structure. Calculated geometry was indicated to belong to a pseudo-S
(Ln = La, Ce, Nd, Sm, Eu, Gd) by referring the analogous crystal structure. Calculated geometry was indicated to belong to a pseudo-S point group with the Ln-N(amine) axis as C
point group with the Ln-N(amine) axis as C rotational axes. Gibbs energy difference indicated that NTAamide ligands more stably coordinate to light or middle Ln ions than La ion. This tendency was comparable to the experimental result of distribution ratio for Ln ions reported previously.
rotational axes. Gibbs energy difference indicated that NTAamide ligands more stably coordinate to light or middle Ln ions than La ion. This tendency was comparable to the experimental result of distribution ratio for Ln ions reported previously.